Biosimilar Market Overview, Growth Driver, Size, Share & Trends 2024
IMARC Group, a leading market research company, has recently releases report titled “Biosimilar Market Size, Share, Trends and Forecast by Molecule, Indication, Manufacturing Type, and Region, 2025-2033”. The study provides a detailed analysis of the industry, including the global biosimilar market trends, share, size and growth forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Report Highlights:
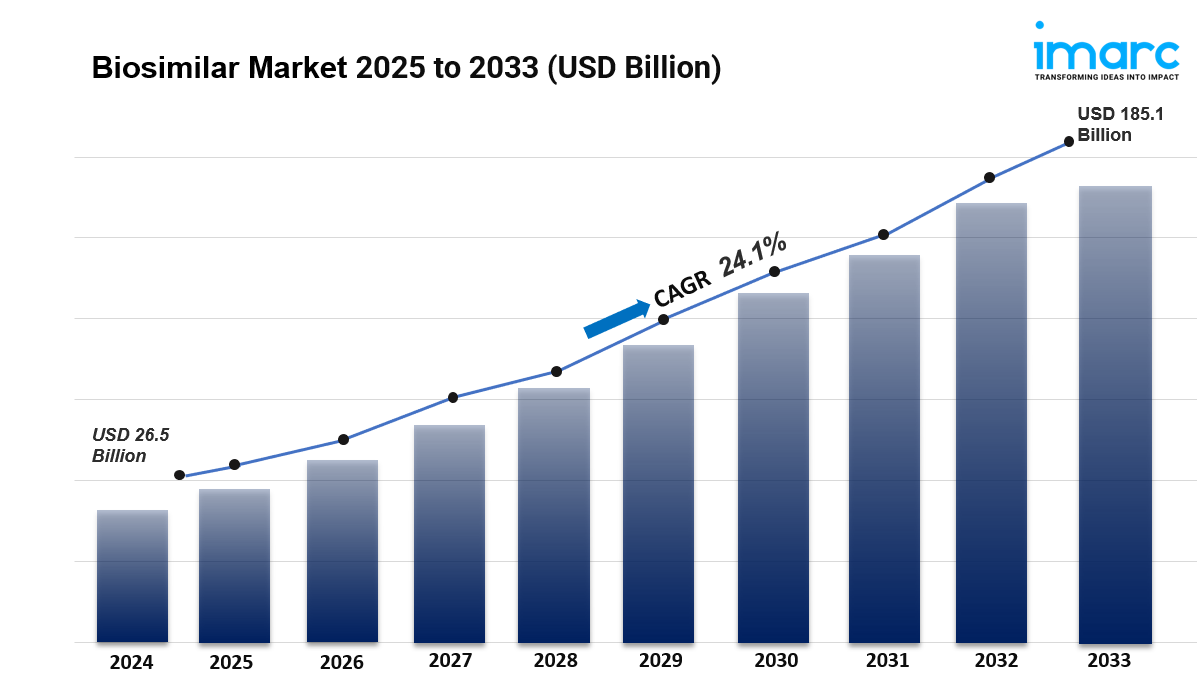
The global biosimilar market size was valued at USD 26.5 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 185.1 Billion by 2033, exhibiting a CAGR of 24.1% from 2025-2033. Europe currently dominates the market. The expiration of patents for major biological drugs, growing awareness about the efficacy and cost-effectiveness of biosimilars, the rising prevalence of chronic diseases worldwide, and continual advancements in biopharmaceutical manufacturing technologies are some of the major factors boosting the biosimilar market share.
Global Biosimilar Market Trends:
The biosimilar market is set for significant growth due to several key factors. By 2025, we expect more biosimilars to gain approval as regulators simplify the approval process. This change will boost competition among manufacturers, which should lower prices and improve access to essential therapies. Healthcare professionals and patients are becoming more accepting of biosimilars. This acceptance will be crucial for expanding their market presence. Educational efforts that explain biosimilars and their safety will help build trust in these alternatives. Moreover, the rise of personalized medicine and targeted therapies is likely to increase demand for biosimilars that fit these trends. As healthcare systems face rising costs, biosimilars offer a practical way to improve affordability without sacrificing care quality. In summary, the biosimilar market in 2025 will showcase a blend of regulatory support, technological progress, and changing healthcare needs, making it a vital part of the future of medicine.
Factors Affecting the Growth of the Biosimilar Market Industry:
Increasing Regulatory Support:
The biosimilar market is changing rapidly. Governments and health authorities worldwide are providing more support. Agencies like the FDA in the U.S. and the EMA in Europe have set clear approval guidelines for biosimilars. This clarity helps speed up development and cuts costs for bringing these products to market. It also boosts confidence among manufacturers and investors. As a result, more pharmaceutical companies are joining the biosimilar space. This leads to more available products. Increased competition is likely to lower prices for patients and healthcare systems. Additionally, as more biosimilars get approved, healthcare providers and patients will likely trust these products more. This trend will further drive market growth.
Rising Demand for Cost-effective Healthcare Solutions:
The rising costs of biologic therapies have created a need for affordable treatments. This demand is driving interest in biosimilars. Healthcare providers and payers want cost-effective alternatives to expensive biologics, especially in areas like oncology, autoimmune disorders, and diabetes. With budget constraints, adopting biosimilars can ease financial burdens while keeping treatment effective. The increasing number of chronic diseases worldwide will likely boost the demand for biosimilars even more. As more patients need long-term care, the savings from biosimilars will attract both providers and patients. This trend may lead to the development of new biosimilar products, broadening treatment options and improving patient access to essential therapies.
Technological Advancements in Biomanufacturing:
Technological advancements in biomanufacturing are shaping the biosimilar market. New production techniques, like continuous manufacturing and better cell line development, boost efficiency and scalability. These improvements lower manufacturing costs and enhance the quality and consistency of biosimilars, making them more competitive with reference biologics. Also, as companies invest in modern biomanufacturing, new players can enter the market more easily. This leads to more competition and innovation. Efficient production methods will speed up the time-to-market for new biosimilars, helping to meet the rising demand for alternative therapies. As the market changes, these tech improvements will be key to the biosimilar sector's growth and sustainability.
Request to Get the Sample Report: https://www.imarcgroup.com/biosimilar-market/requestsample
Biosimilar Market Report Segmentation:
Breakup By Molecule:
- Infliximab
- Insulin Glargine
- Epoetin Alfa
- Etanercept
- Filgrastim
- Somatropin
- Rituximab
- Follitropin Alfa
- Adalimumab
- Pegfilgrastim
- Trastuzumab
- Bevacizumab
- Others
Infliximab accounts for the majority of shares due to its widespread use in treating chronic autoimmune diseases such as rheumatoid arthritis and Crohn's disease.
Breakup By Indication:
- Auto-Immune Diseases
- Blood Disorder
- Diabetes
- Oncology
- Growth Deficiency
- Female Infertility
- Others
Autoimmune diseases dominate the market as biologics, and biosimilars are highly effective in managing conditions like rheumatoid arthritis, psoriasis, and inflammatory bowel disease.
Breakup By Manufacturing Type:
- In-house Manufacturing
- Contract Manufacturing
In-house manufacturing represents the majority of shares because it enables better control over production quality and reduces reliance on third-party manufacturers, ensuring compliance with strict regulatory standards.
Breakup By Region:
- Europe
- Germany
- France
- Italy
- Spain
- United Kingdom
- Rest of Europe
- United States
- Japan
- India
- South Korea
- Rest of the World
Europe holds the leading position due to its well-established regulatory pathways for biosimilars and strong government support for biosimilar adoption.
Top Biosimilar Market Leaders:
The biosimilar market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies.
Some of the key players in the market are:
- Sandoz International GmbH
- Pfizer Inc.
- Teva Pharmaceutical Industries Limited
- Celltrion Inc.
- Biocon Limited
- Samsung Biologics
- Amgen, Inc.
- Dr. Reddy's Laboratories Limited
- Stada Arzneimittel Ag.
Speak to An Analyst: https://www.imarcgroup.com/request?type=report&id=497&flag=C
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145
- Local News
- World News
- Crime
- Politik
- Film
- FootBall
- Food
- Jeux
- Health
- Domicile
- Literature
- Music
- Networking
- Autre
- Religion
- Shopping
- Sports
- Opinion
- Tech
- Scam
- Bussines News
- Credit
- Hosting
- Insurance
- Infomation
- Finance
- Entertaiment
- Éducation
- Artist
- Trick and hack
- Forex
- Aperçu
- Vps Forex
- Cerita
- agriculture
- assistance


